Third quarter 2024. The Lab Medicine Update is a collaboration between the UVA Medical Laboratories and the UVA Clinical Laboratory Stewardship Subcommittee. Feedback on the LMU? Contact Jim Harrison (james.harrison@virginia.edu).
Flow Cytometry Anti-CD20 Panel Reporting ↑
The FLOW anti-CD20 panel was developed for monitoring patients on anti-CD20 immunotherapy such as Rituximab. The results reported from this assay are CD19+ lymphocytes and CD20+ lymphocytes as a percentage of total lymphocytes. Reference ranges are not provided. Percentages are reported to the nearest whole number. This assay is not designed to detect leukemia/lymphoma and no pathologist consultation is provided.
INTERCEPT® Fibrinogen Complex (IFC) for STAT Cryoprecipitate from the Blood Bank ↑
INTERCEPT® Fibrinogen Complex (IFC) is a pathogen-reduced unit of cryoprecipitate (cryo) that is a replacement for a unit of regular cryo. It has the advantage of a thawed shelf life of 5 days (rather than 4 hrs for cryo), making it more readily available for emergencies that require cryo. Beginning August 19, the Blood Bank will have IFC thawed and ready to issue on an ongoing basis when cryo is ordered STAT. The Blood Bank will determine when it is appropriate to send IFC for a cryo order (IFC cannot be ordered specifically). There will be no changes to ordering cryo (Epic) or administering cryo/IFC (2-person time-out plus BPAM). A unit of IFC looks the same as a unit of cryo except for the component information as shown below.

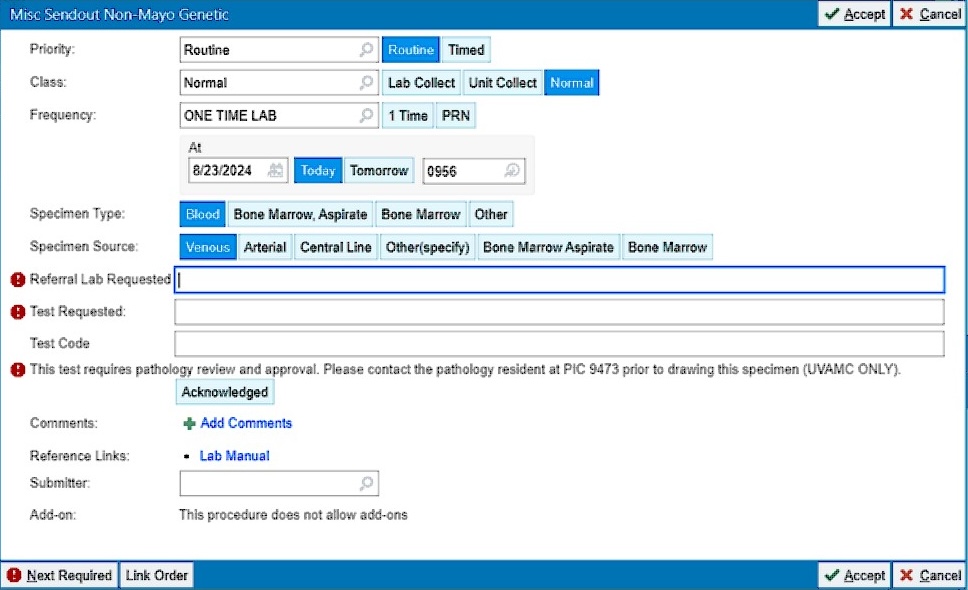
Update to Miscellaneous Sendout Orders ↑
Additional required fields have been added to the Miscellaneous Sendout Non-Mayo Genetic (LAB3923) and Misc Sendout Non-Mayo Non-Genetic (LAB3927) order panels. When ordering these tests LIPs should now enter the referral lab requested, the test requested, and the test code (see red exclamation signs in the image below). These data are needed to clearly indicate the specific test requested and lab to which the samples should be sent. These data will improve the efficiency and speed of handling sendout testing, and will decrease the number of callbacks to LIPs for clarification.
BioFire FilmArray Meningitis/Encephalitis (ME) Panel ↑
Effective July 23, 2024 the UVA Microbiology and Molecular Diagnostics Laboratory will begin performing the BioFire FilmArray Meningitis/Encephalitis (ME) Panel (previously available only as a send out test). Due to the complexities and limitations of this assay, testing will only be performed with approval of the Microbiology Director on call. Please page PIC 1221 prior to submission of the specimen to prevent delays in testing.
The BioFire ME Panel is a qualitative multiplexed nucleic acid-based test for detection and identification of multiple bacterial and viral nucleic acid targets directly from cerebrospinal fluid (CSF) specimens obtained via lumbar puncture from individuals with signs and/or symptoms of meningitis and/or encephalitis. Testing from all other collection methods will be rejected.
The following organisms are identified using the BioFire ME Panel:
| Bacteria | Viruses |
|---|---|
| Escherichia coli K1 | Cytomegalovirus |
| Haemophilus influenzae | Enterovirus |
| Listeria monocytogenes | Herpes simplex virus 1 and 2 |
| Neisseria meningitidis (encapsulated) | Human herpesvirus 6 |
| Streptococcus agalactiae | Human parechovirus |
| Streptococcus pneumoniae | Varicella zoster virus |
Limitations and/or Special notes regarding testing with the BioFire ME Panel:
- Results should be used in conjunction with other clinical, epidemiological, and laboratory data, and is not intended to be used as the sole basis for diagnosis, treatment, or other patient management decisions.
- Positive results do not rule out co-infection with organisms not included in the test. The agent detected may not be the definitive cause of disease. Negative results do not preclude central nervous system (CNS) infection. Not all agents of CNS infection are detected by this test and sensitivity in clinical use may vary.
- This test is not a replacement for CSF culture for at-risk patients. Testing for cryptococcal meningitis is not included. Order Cryptococcal Antigen [Lab927] for detection of Cryptococcus neoformans/gattii. Test results should be interpreted in the context of host factors and other laboratory information.
- Sensitivity of this test for HSV 1 and 2 is suboptimal. Testing by another molecular method (LAB6153) is recommended if clinical suspicion is high.
- Latent reactivation or shedding of herpesviruses (CMV, HHV-6, HSV-1, HSV-2, and VZV) in CSF may be detected with this test. Results should be interpreted in the appropriate clinical context.
- Non-K1 E. coli serotypes may be present in a specimen and will not be detected by this test.
- Non-encapsulated strains of Neisseria meningitidis are not detected by this test.
- This test cannot distinguish between latent and active CMV and HHV-6 infections. Detection of these viruses may indicate primary infection, secondary reactivation, or the presence of latent virus. Results should always be interpreted in conjunction with other clinical, laboratory, and epidemiological information.
- Viral shedding into the CSF often occurs in cases of zoster (shingles; caused by reactivation of VZV). Detection of VZV in CSF may not indicate the cause of CNS disease in these cases.
New Collection Device for Chlamydia/Gonorrhea Testing ↑
The UVA Clinical Microbiology and Molecular Diagnostics laboratory has implemented a new method for molecular detection of Chlamydia and Gonorrhoeae, which requires a new collection device. Please ensure samples are collected using the BLUE “Alinity-m Multi-Collect Specimen Collection Kit” circled in the photograph below.

- There is no change to the sample collection method. Only the collection device has changed.
- Only the orange shaft swab provided in the BLUE specimen collection kit should be used.
- Do NOT discard the media in the tube when inserting swab or filling with a urine sample.
- Acceptable specimens include:
- Clinician-collected vaginal, endocervical, oropharyngeal or rectal swab specimens
- Male or female urine samples
- Self-collected vaginal swabs (if collected in a healthcare setting)
- Samples collected with the previously used Abbott Multi-Collect specimen collection kit in WHITE packaging, will be batched and tested only once weekly, which may cause significant delays in result reporting.
- If the WHITE collection device is used and the extended turnaround time is unacceptable, the sample will need to be recollected with the BLUE collection device.
Per our Supply Chain colleagues, these new collection kits have been rolled out to all university hospital units and UVA Clinic locations. If you do not have the BLUE Alinity-m Multi-Collect Specimen Collection Kit, please contact Supply Chain to request this product immediately.
For questions or concerns, please contact the Clinical Microbiology director on call at PIC 1221.